A Absorbance. Beer -Lambert Law.

Spectrophotometry And Beer Lambert Law Youtube
A εbc where ε is.

. ε Molar absorption coefficient Molar absorptivity or Molar extinction coefficient. Beer Lambert Law states that the transmission of a monochromatic light through a substance is logarithmetically related to the product of the concentration path length distance that light travels through the material and the molar extinction coefficient of the substance. You will be applying Beers law to calculate the concentration.
This is used as stock solution. Divide the mass of the solute by the total volume of the solution. L whereIoand Itare the incident and transmitted intensitiesA absorbance and a constant absorptivity formerly called the extinction coefficientIf the concentration is measured in molL-1 the absorptivity is called the molar absorptivity.
The equation for Beers law is a straight line with the general form of y mx b. Concentration Path-length and Absorbance Calculator. The Beer Lambert law defines the relationship between the concentration of a solution and the amount of light absorbed by the solution.
C l or c A. To find an unknown concentration we. The equation for Beers law is.
Determine the molar absorption coefficient of the solution. Concentration Path-length and Absorbance Calculator. Thus l o g 1 l o g I t 0 l o g I t 00376 x 8 x 2 06016.
Find the concentration of the solution. A E l C. Using this law any of the four properties can be calculated by.
Where A is the absorbance. In this case use the absorbance found for your unknown along with the slope of your best fit line to determine c the concentration of the unknown solution. The second method is the calibration curve which is also principally based on Beer-Lamberts law.
How Beers law determine concentration of a solution. Plug in the values you found for the mass and volume and divide them to find the concentration of your solution. 070 8400 M-1cm-1 1cm c By dividing both sides of the equation by 8400 M-1cm-1 1cm c 833 x 10-5 molL.
C is the concentration and l is the cells width E epsilon coefficient and its unit is moldm3. Write out the equation C mV where m is the mass of the solute and V is the total volume of the solution. What is the unit of absorbance According to Beer-Lambert law.
So according to the Beer-Lambert law absorbance equals epsilon times length of container or the length that the light has to travel through to pass through the solution times concentration. Calculate the concentration of the sample. The graph should plot concentration independent variable on the x-axis and absorption.
This video shows how to use the Beer-Lambert law to calculate the concentration c for a sample when the absorbance extinction coefficient and path length. The molar absorptive is 120 Lmol cm and the path length is 10 mm. Steps to calculate absorption from Beer-Lambert Law.
Therefore I t 02503 25. A εmCl Aabsorbance εm molar extinction coefficient C concentration lpath length of 1 cm You should have a data set which was used to create a standard curve. Calculate or measure the percent transmittance of solutions at different concentrations using Beer Lambert law or Beers law calculator online.
A εlc Note. A spectrophotometer value detected A070. The absorbance of a sample is 016.
Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. Beer -Lambert Law. You will be applying Beers law to calculate the concentration.
Since we know ϵ we can calculate the transmission using Beer-Lambert Law. The absorption coefficient of a glycogen-iodine complex is 020 at light of 450 nm. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration.
By using Beers law we will calculate the concentration of the sample. The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation. L Path Length.
What does the Beer. Please show work for points. C Concentration.
Practice Using the Beer-Lambert Law to Calculate the Concentration of a Solution with practice problems and explanations. ε will changed when the weave length changed. From the above stock solution prepare five different concentrations of KMnO4 solutions say 00001 M 000025 M 00005 M 000075 M and 0001 M.
Get instant feedback extra help and step-by. Prepare a standard 01 M aqueous KMnO4 solution. Calculate the length of the path in which the light beam travels.
Use the Beer-Lambert Law to calculate concentration given the following information. The equation to be used Beer-Lambert Law is. According to Beer-Lambert law log Io It A.
Generally l is constant 1 CM. If you know the absorption coefficient for a given wavelength and the. How do you find the concentration of an unknown solution.
Finally calculate absorption using the formula mentioned above. Beer-Lambert law Using available information of any standard solution to determine the ε Then using these information to get the unknown concentration using. Where the slope m is equal to εl.
L o g I t -06016.

Beer Lambert Law Concentration Youtube
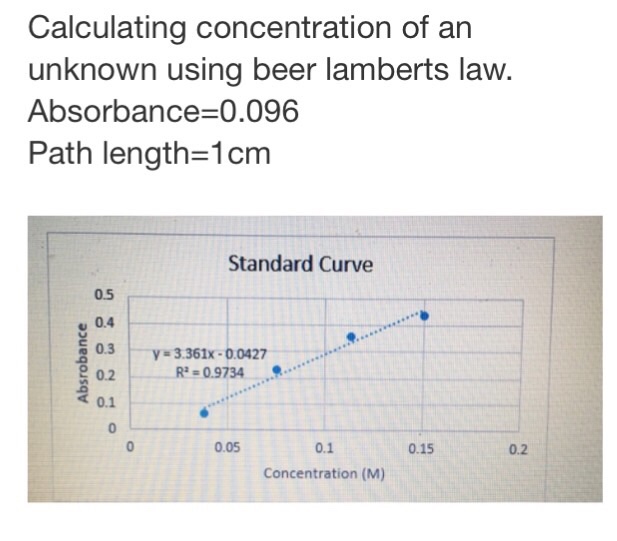
Solved Calculating Concentration Of An Unknown Using Beer Chegg Com

How To Find Molar Absorptivity Using The Beer Lambert Law Chemistry Study Com
0 Comments